 |
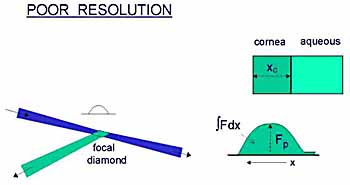
I suggest that a better approach would be to derive the
total amount of fluorescein in the cornea from the area under the corneal
concentration profile, as carried out in the tears by Joshi et al.
Deconvolution of the profile would seem to be another alternative, but I have no experience with it. |
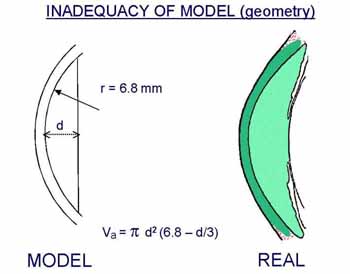 |
The importance of false assumption as to the geometry of the model depends on the purpose of the experiment, in particular, whether comparative or absolute flow values are required. |
 |
The effect of exchanges with other compartments has been dealt
with by Brubaker, and how to correct for changes in diffusional loss across
the iris was explained by Anselmi et al.
Poor mixing in the anterior chamber is a nuisance that can be overcome by making eye movements. |


