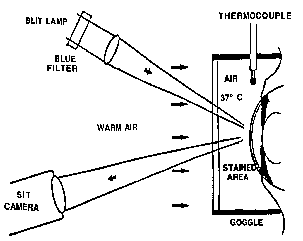
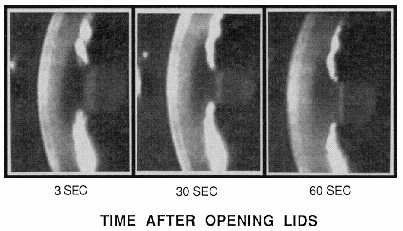
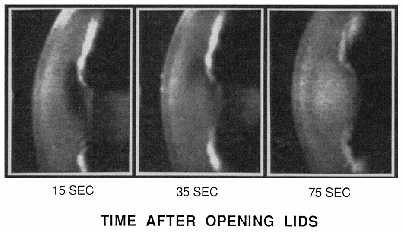
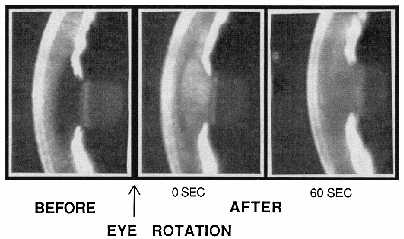
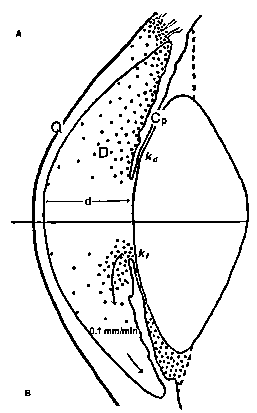
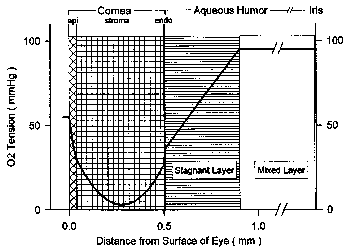
| References
Aserinsky, E. and Kleitman, N. (1953). Regularly occurring periods of
eye motility, and concomitant phenomena, during sleep. Science 118, 273-4.
Crick, F. and Mitchison, G. (1983). The function of dream sleep. Nature
304, 111-14.
Cunha-Vaz, J. and Maurice, D. (1969). Fluorescein dynamics in the eye.
Doc. Ophthalmol. 26, 61-72.
Duke-Elder, W. S. (1958). The eye in evolution. Vol 1. System of ophthalmology.
(Duke-Elder, W. S., ed.). Mosby: St Louis, U.S.A.
Dutton, J. J. (1996). Botulinum-A toxin in the treatment of craniocervical
muscle spasms: short- and long-term, local and systemic effects. Surv.
Ophthalmol. 41, 51-65.
Easter, S. S. (1971). Spontaneous eye movements in the restrained goldfish.
Vis. Res. 11, 333-42.
Ehrlich, P. (1882). Uber provozierte Fluorescenz- Erscheinungen am Auge.
Deutsch. Med. Wochenschr. 8, 35-7.
Fatt, I. and Weissman, B. A. (1992). Physiology of the eye. 2nd. Ed.
Butterworth, Heinmann: Boston, U.S.A.
Fatt, I., Freeman, R. D. and Lin, D. (1974). Oxygen tension distributions
in the cornea. A re-examination. Exp. Eye Res. 18, 357-65.
Hobson, A. (1990). Ontogenetic development of the human sleep-dream
cycle. Neuroscience 10, 371-82.
Hoffert, J. R. and Fromm, P. O. (1969). Viscous properties of teleost
aqueous humor. Comp. Biochem. Physiol. 28, 1411-17.
Holm, O. (1968). A photogrammetric method for estimation of the pupillary
aqueous flow in the living human eye. Acta Ophthalmol. 46, 254-83.
Koch, J. M., Refojo, M. J., Leong, F. and Kenyon, K. R. (1989). Keratopathy
of the rabbit cornea following complete eyelid closure. Acta Ophthalmol.
67, Suppl. 192, 108-13.
Lavie, P. (1996). The enchanted world of sleep. (Donnelley, Harrisonburg).
Maurice, D. M. (1967). Nutritional aspects of corneal grafts and prostheses.
In: Corneo-plastic surgery. (Ed. Rycroft, P.) (Pergamon, Oxford).
Maurice, D. M. (1984). The cornea and sclera. In The eye Vol 1B, ed.
Davson, H. 3rd Edition. Academic: New York.
Maurice, D. M. (1995). The effect of the low blink rate in rabbits on
topical drug penetration. J. Ocul. Pharm. Thera. 11, 297-304.
Narebski, J., Tymicz, J., and Lewosz, W. 91969). The circadian sleep
of rabbits. Acta Biologicae Experimentalis. 29, 185-200.
Okawa, M., Nanami, T., Wada, S., Shimizu, T., Hishikawa, Y., Sasaki,
H., Nagamine, H. and Takahashi, K. (1987). Four congenitally blind children
with circadian sleep- wake rhythm disorder. Sleep 10, 101-10.
Pettigrew, J. D., Wallman, J. and Wildsoet, C. F. (1990). Saccadic oscillations
facilitate ocular perfusion from the avian pecten. Nature 343, 362-3.
Pivik, R. T., Bylsma, F. W. and Cooper, P. (1986). Sleep wakefulness
rhythms in the rabbit. Behav. Neuro. Biol. 45, 275-6.
Riley, M. V. and Winkler, B. S. (1990). Strong Pasteur effect in rabbit
corneal endothelium preserves fluid transport under anaerobic conditions.
J. Physiol. 426, 81-93.
Roffwarg, H. P., Muzio, J. N. and Dement, W. C. (1966). Ontogenetic
development of the human sleep-dream cycle. Science 152, 604-19.
Smelser, G. K., Ozanics, V. (1953). Structural changes in corneas of
guinea pigs after wearing contact lenses. Arch. Ophthalmol. 49, 335-40.
Snyder, F. (1966). Towards an evolutionary theory of dreaming. Amer.
J. Psychiat. 123, 131-6.
Türk, S. (1906). Untersuchungen über eine Strömung in
der vorderen Augenkammer. v. Graefe's Arch. Ophthalmol. 64, 481-501.
Weiss, T. and Roldan, E. (1964). Comparative study of sleep cycles in
rodents. Experientia 20, 280-1.
Wincek, J. and Ruttum, M. S. (1989). Exposure keratitis in comatose
children. J. Neurosci. Nursing 21, 241-4.
Wyatt, H. J. (1996). Ocular pharmacokinetics and conventional flow.
J. Ocul. Pharm. Therapeut. 12, 441-59.
Zepelin, H. (1989). Mammalian sleep. In: Principles and practice of
sleep medicine. (Ed. Kryger, M. H., Roth, T. and Dement, W. C.) Saunders:
Philadelphia.
|